Browse by Category
Featured Products

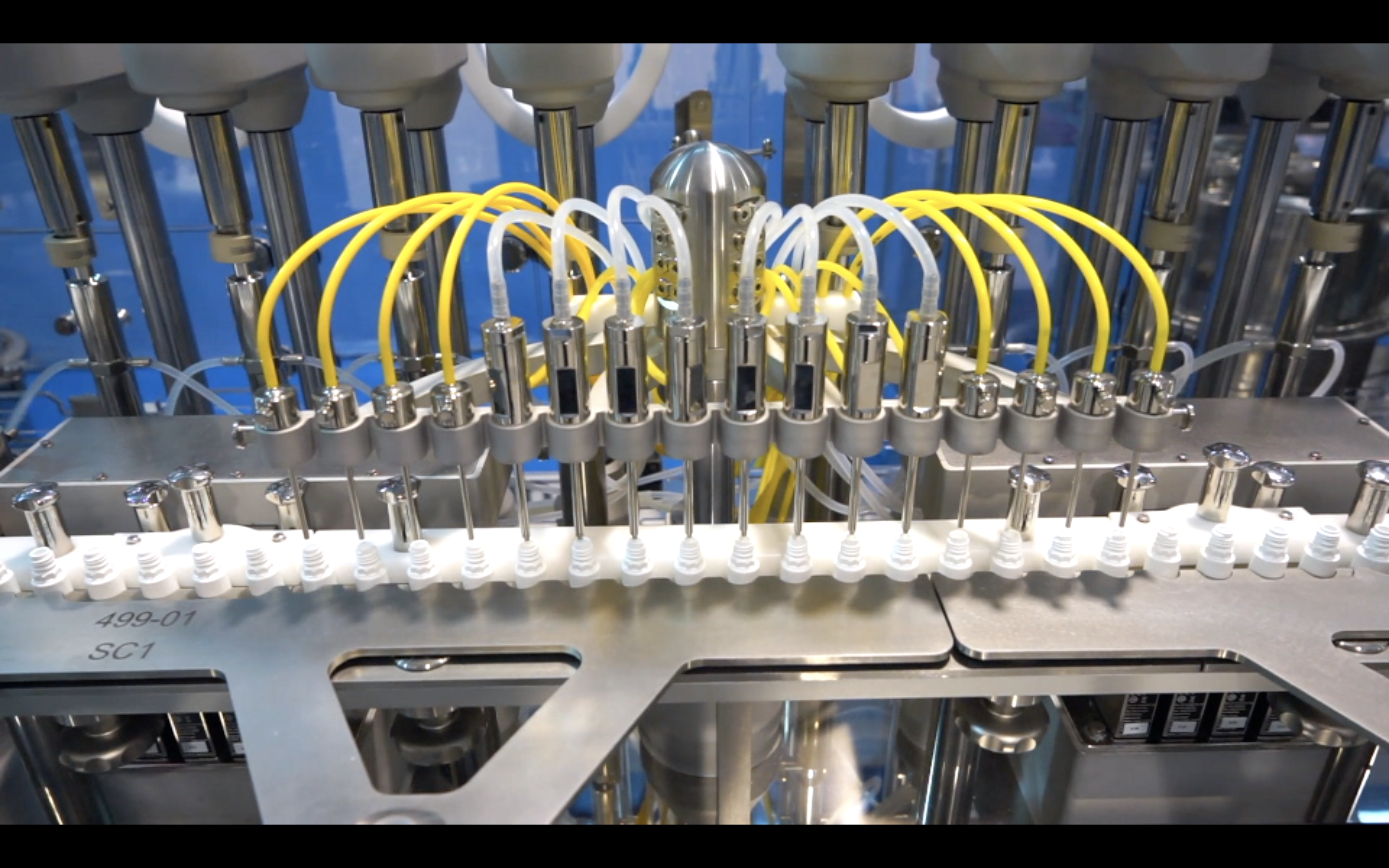
SP i-Dositecno Versa-Line Pharmaceutical Filling Equipment
SP i-Dositecno LI Versa-Line™ Pharmaceutical Filling Equipment
LINE OF SIGHT ENABLED

SP Hull LyoConstellation S10/S20/S30 Pilot Lyophilizers
SP Hull LyoConstellation™ S10/S20/S30 Pilot Lyophilizers – a s…

SP Genevac EZ-2 4.0 Series Centrifugal Evaporators
Three models: Standard, Plus, Elite to suit your needs

SP VirTis Genesis Pilot Freeze Dryer
Three models: Standard, Plus, Elite to suit your nSP VirTis Genesis Pilot Freeze…
LINE OF SIGHT ENABLED

SP Hull LyoStar 4.0 R&D/Process Development Freeze Dryer
SP Hull LyoStar® 4.0 R&D and Process Development Freeze …

SP Hotpack SpaceSaver™ Glassware Washers
The only way is up! Get maximum impact with minimal disrupti…

Our People Make the Difference
Our SP service team offers global expert
service and a range of support packages.
Discover how our customizable service
packages can help you maximize uptime,
improve productivity, and increase
longevity of your investments.

Learn from Industry Leaders
Explore on-demand webinars, technical papers and articles, the latest training offerings and much more.

Industry Leading SP Brands
Whether you’re in the pharmaceutical, life science, or a related market, you’ll be sure to find the right products in our broad portfolio of SP brands and products covering biopharma manufacturing and life science equipment, and a comprehensive range of labware and glassware products.
Need More Help?
We’d love to help you find the perfect solution for your problem. Give us a call or reach out through our contact form. US +1-800-431-8232